Plant-Pathogen Interactions
Research Groups
Plant Immune Design GroupStaff
- Asst. Prof. Dr. FUKADA Fumi
- E-mail:fumi.fukada@(@okayama-u.ac.jp)
- Plant pathology, Plant-microbe interaction
Research Topics
Earth's population is expected to reach 9 billion by 2050. Plant agriculture must change fundamentally to support this number of people by mid-century. Rice is the most important food crop and therefore, improvement of rice is a key research goal. Our ultimate goal is to design new rice plants to cope with biotic and abiotic stresses and improve important agronomic traits. We have been working on immune receptors and the small GTPaseOsRac1, which are major components of rice immunity. A comprehensive understanding of immune receptors and OsRac1 functions would allow the design of rice immune system to control pathogens. We are also interested in how plants orchestrate epigenetic regulations in responding to abiotic stresses. To address these issue, we have been investigating rice as a model system with a combination of several cutting-edge technologies including live imaging and gene editing.
Involvement of phytocytokines in rice immunity
|
Plants cleverly regulate their immunity by secreting endogenous peptides called plant cytokines (phytocytokines). We believe that understanding and optimizing phytocytokine signaling and their networks will improve plant immunity, and we are conducting this research. Recently, we have established a multi-omics pipeline to isolate potential phytocytokines in rice and identified 236 endogenous secreted peptides that were induced during the disease resistance response (Plant Biotech J 2020). These included cytokine-like peptides such as the cysteine-rich RALF7, the post-translationally modified PSK4, and a novel family of IRPs that do not contain any known domains. Further analysis revealed that IRPs do indeed have cytokine activity and act as positive regulators of resistance to blast fungus, the most important pathogen of rice (bioRxiv 2021). 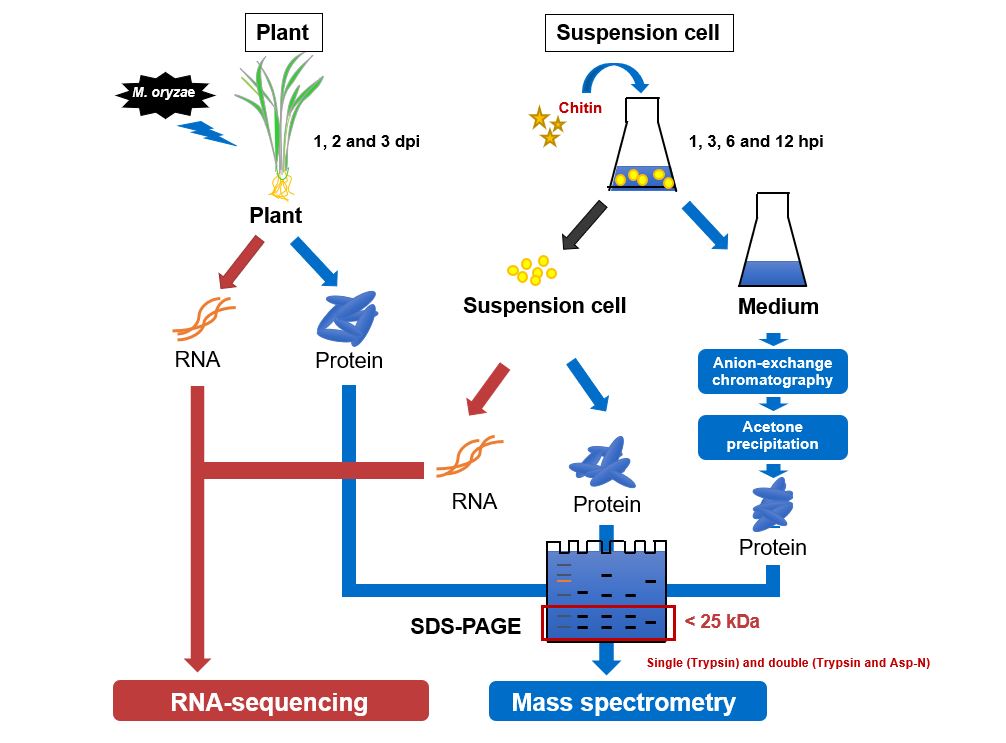
|
Effector-triggered immunity through small GTPase OsRac1
|
Resistance (R) proteins are crucial intracellular receptors that detect attacks by insects and invasion by various pathogens, including fungi, bacteria and viruses. However, the signaling molecules which mediate R protein-induced immune responses are not yet fully understood. We have previously shown that an intracellular switch, the small GTPase OsRac1, is a master regulator controlling immunity in rice (Plant Cell 2016, Curr Genomic 2016, FPS 2014, COPB 2013, Rice 2010). However, the mechanism by which OsRac1 receives signals from the immune receptors and becomes activated has remained unclear. Thus, we explored OsRac1-binding proteins and identified the R protein Pit, which is an immune receptor for rice blast fungus, a prominent microbial disease of rice. Through various analyses, we demonstrated that OsRac1 functions as a molecular switch, controlling ROS production and hypersensitive cell death (CHM 2010) and a GDP/GTP exchanger OsSPK1 mediates the activation of OsRac1 by Pit1 (PNAS 2018). Besides, we have found that anchoring Pit to the plasma membrane through palmitoylation, a type of lipid modification, is required for Pit-induced activation of OsRac1 on the plasma membrane (JBC 2014). Our work has therefore revealed the signaling pathway of the R protein Pit through OsRac1. 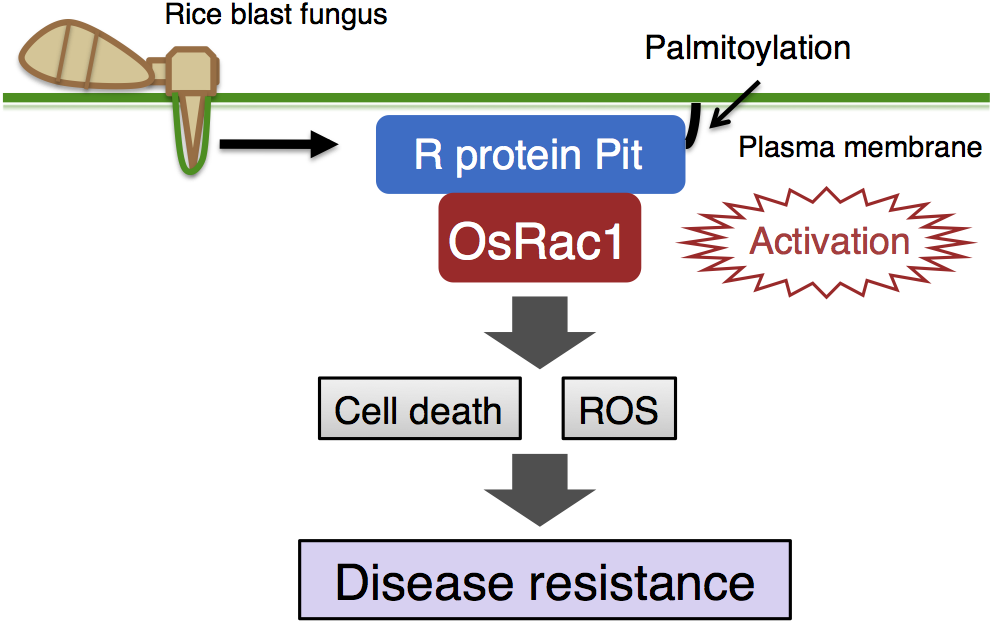
|
Pattern-triggered immunity through small GTPase OsRac1
|
Given that the small GTPase OsRac1 is a master regulator controlling rice immunity, monitoring its activation within plant cells was believed to be the next key step in understanding plant immunity. By creating a bio-imaging sensor using the Forster resonance energy transfer (FRET) of fluorescent proteins, we were the first to successfully monitor the activation of a plant small GTPase in vivo (CHM 2010; Plant Method 2018). Using this sensor, we observed that OsRac1 is activated within 3 min after sensing chitin, a cell wall component of rice blast fungus (CHM 2013). Furthermore, we revealed that the signal from the chitin receptor OsCERK1 is transmitted to OsRac1 through the OsRac1 activator protein OsRacGEF1. We have also reported that OsRac1 controls the expression of defense-related genes through the MAPK OsMPK6 and the transcription factors RAI1 and Rap2.6 (PCP 2012; Rice 2012). We have also identified a number of endogenous peptides involved in PAMP-induced immunity (Plant Biotech J, 2020). 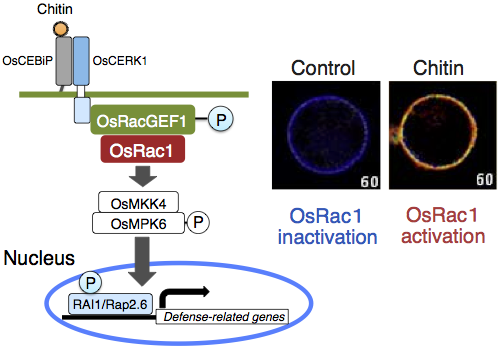
|
Publication List
- Wang, Q., Li, Y., Kosami, K., Liu, C. Li, J., Zhang, D., Miki, D., Kawano, Y. Three highly conserved hydrophobic residues in the predicted α2 helix of rice NLR protein Pit contribute to its localization and immune induction Plant Cell Environ 2022 in press
- Kawano Y. Fine-tuning ROS homeostasis by ROD1 is a battleground between rice and Magnaporthe oryzae Mol Plant 2021 14(12):1979-1981.
- Akamatsu, A., Fujiwara, M., Hamada, H., Wakabayashi, M., Yao, A., Wang, Q., Kosami, K., Dang, T.T., Kaneko-Kawano, T., Shimamoto, K., Kawano, Y. The Small GTPase OsRac1 forms two distinct immune receptor complexes containing the PRR OsCERK1 and the NLR Pit Plant Cell Physiol. 2021 62(11):1662-1675
- Fukada F, Rössel N, Münch K, Glatter T, Kahmann R. A small Ustilago maydis effector acts as a novel adhesin for hyphal aggregation in plant tumors. New Phytol 2021 231(1):416-431
- Jing, Z., Wacera, W F., Takami, T., Takanashi, H., Fukada, F., Kawano, Y., Kajiya-Kanegae, H., Iwata, H., Tsutsumi, N., Sakamoto, W. NB-LRR-encoding genes conferring susceptibility to organophosphate pesticides in sorghum. Sci Rep. 2021 11(1):1982
- Wang. P. #, Yao, S. #, Kosami, K., Zhang, Y., Fukao, Y., Zhang, H., She, Y.M., Hanada, K., Liu, R.*, and Kawano, Y. *Identification of endogenous small peptides involved in rice immunity through transcriptomics- and proteomics-based screening. Plant Biotech J 2020 20120.doi: 10.1111/pbi.13208
- Fukada F, Kodama S, Nishiuchi T, Kajikawa N, Kubo Y. Plant pathogenic fungi Colletotrichum and Magnaporthe share a common G1 phase monitoring strategy for proper appressorium development. New Phytol 2019 222(4):1909-1923
- Tanaka S, Schweizer G, Rössel N, Fukada F, Thines M, Kahmann R.Neofunctionalization of the secreted Tin2 effector in the fungal pathogen Ustilago maydis. Nat Microbiol 2019 Feb;4(2):251-257 2018
- Wang, Q. , Li, L., Ishikawa, K., Kosami, K., Uno, K., Nagawa, S., Tan, L., Du, J., Shimamoto, K., and Kawano, Y. Resistance protein Pit interacts with the GEF OsSPK1 to activate OsRac1 and trigger rice immunity. PNAS 2018 115(49):E11551-E11560
- Xie Y., Zhang Y., Han J., Luo J., Li G., Huang J., Wu H., Tian Q., Zhu Q., Chen Y., Kawano, Y., Liu Y.-G., and Chen L. The Intronic cis-Element SE1 Recruits trans- acting Repressor Complexes to Repress the Expression of ELONGATED UPPERMOST INTERNODE1 in Rice. Mol Plant 2018.doi: 10.1016/j.molp.2018.03.001
- Wong, HL., Akamatsu, A., Wang, Q., Higuchi, M., Matsuda, T., Okuda, J., Kosami, K., Inada, N., Kawasaki, T., Nagawa, S., Tan, L., Kawano, Y. (corresponding author), Shimamoto, K. In vivo monitoring of plant small GTPase activation using a Förster resonance energy transfer biosensor Plant Methods 2018 14:56