Research Area : Applied Natural Product Chemistry
Investigation of inhibition mechanism of sialidase of influenza virus and development of novel inhibitors: difluorosialic acid and sulfo-sialic acid
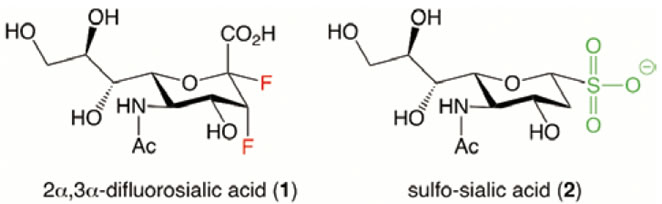
Development of improved influenza sialidase inhibitors is critical to prepare for potential influenza pandemics. Based on the hypothesis that Tyr406 forms an acetal intermediate in the catalytic cycle of sialidase, we synthesized 2a,3a-difluorosialic acid (1) as a suitable probe to test if a covalent intermediate can be captured. As expected, X-ray crystallographic analysis revealed that 1 forms a covalent complex with Tyr406 of influenza silalidase. This indicated that Tyr406 attacks from the b-face of the substrate, leading to hydrolysis with retention (inversion and inversion) of the configuration. In addition, 1 possessed potent anti-influenza activity (including that of Tamiflu resistant virus) with IC50 inhibitory constants of 10 to 1000 nm, providing the first proof-of-concept for mechanism-based influenza sialidase inhibition.
In addition, a next-generation inhibitor, sulfo-sialic acid (2), was developed. The sialidase inhibitory activity of 2 was more potent than the corresponding carboxy and phosphono derivatives, presumably due to stronger attraction to the triarginyl binding site of the enzyme.